everything we're up to
our research
what do we do?
we gave up all our free time for this
We study B-cell antibody responses to HIV, influenza, and the new SARS-CoV-2 virus with the goal of understanding how antigenic sites of vulnerability are initially perceived by specific germline B-cell receptor sequences, and how that recognition process can direct antibody affinity maturation toward production of broadly neutralizing anti-viral responses.
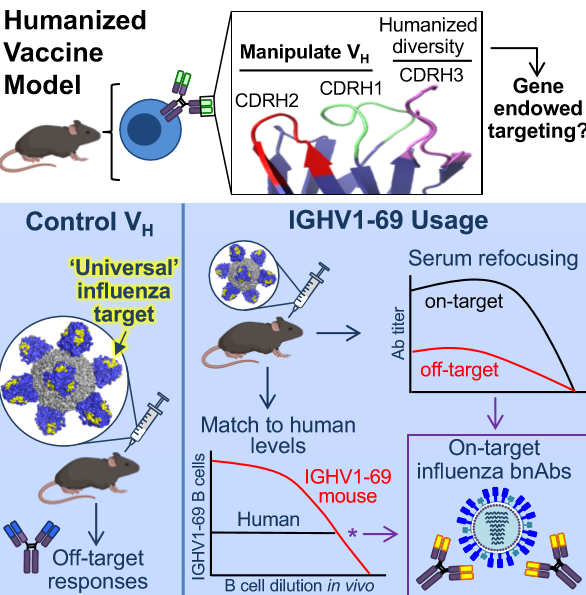
Our research centers on defining how germline B cell antigen recognition controls epitope targeting in the downstream antibody response. We focus on how gene-encoded ‘pattern’ recognition motifs within the antibody repertoire naturally support recognition of normally ‘difficult to see’ vaccine targets on HIV and influenza virus.
To test this, we deploy humanized mouse vaccine models in which we can experimentally vary antibody V gene input to an otherwise fully diverse humanized BCR repertoire. With this system, we can systematically evaluate the antibody gene encoded-contribution to the formation of an antibody paratope, all as a function of immune challenge.
In the influenza space, we have found that specific antibody V gene usage can equate to innate-like B cell recognition of a conserved site of viral vulnerability, as displayed by the intact virion and also recombinant influenza antigens. This gene-encoded targeting principle enables vaccine-elicitation of broadly neutralizing antibodies against flu, a principle we now leveraging for ‘universal’ vaccine design.